Abstract
Introduction: The BCL2 inhibitor venetoclax is approved for the treatment of relapsed/refractory del17p CLL, and has shown activity in patients failing kinase inhibitors (KI). Data on outcomes, toxicities, and dose ramp-up when venetoclax is used in the real world and on treatment selection following venetoclax discontinuation are not available. We aimed to analyze these data in 204 venetoclax-treated patients. To our knowledge, this is the largest series of venetoclax treated patients reported to date.
Methods: We conducted a retrospective cohort analysis of CLL patients treated with venetoclax across 20 US and international academic and community centers. We examined demographics, clinical/genetic prognostic factors, venetoclax dose escalation and long term dosing, tumor-lysis syndrome (TLS) prophylaxis and outcomes, reported adverse events (AEs), overall response rates (ORR), complete responses (CR), survival outcomes, and practice patterns following venetoclax discontinuation. TLS risk was defined as per the venetoclax FDA label. TLS events were defined as per Howard criteria. The primary endpoint was progression-free survival (PFS) measured from the time of venetoclax initiation until progression, death, or last follow up as determined by the Kaplan Meier method. Comparisons of outcomes data were made using the LR test or COX regression. All other comparisons were descriptive.
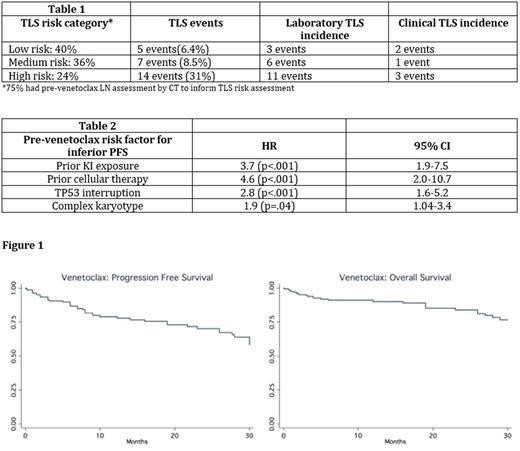
Results: A total of204 CLL patients were included in this analysis (98% relapsed/refractory). Median age at venetoclax initiation was 67 years (range 37-91), 70% males, 89% Caucasians, 73% Rai stage ≥2, median prior therapies was 3 (0-11), 44% del17p+, 28% del11q+, 41% TP53 mutation+, and 33% complex karyotype (≥ 3 abnormalities). Moreover, 27% were NOTCH1 mutated (n=73 tested), 27% BTK mutated (n=51), 10% PLCγ2 mutated (n=49), and 81% unmutated IGHV (n=102). Notably, prior to venetoclax initiation, 64% received prior KI, 15% ≥ 2 KIs, 5.4% cellular therapy (CAR-T or transplantation), 4% second generation KI, 4% prior alternate BCL-2 inhibitor. Median time from most recent therapy to starting venetoclax was 2 months (range: 0-167). For TLS prophylaxis, 93% received allopurinol, 92% normal saline, and 45% rasburicase. 83% of patients had ≥ 1 planned hospitalization for monitoring during ramp up. Data on TLS risk stratification and TLS events are included in Table 1. Dose escalation to the maximum recommended dose of 400 mg daily was achieved in 84% of patients. Following dose escalation, the median stable venetoclax daily dose was 400 mg with 22% requiring a stable dose of < 400 mg daily. At least 1 dose interruption was recorded in 26% of patients (median 2 days, range 1 - 94 days). AEs of interest included neutropenia in 48% (ANC < 1000), thrombocytopenia in 27% (<50k), diarrhea in 14% (>7 bowel movements/day), and neutropenic fever in 9.4%. ORR was 77% (28% CR, median time to best response 3 months). In patients with either BTK or PLCγ2 mutations (n=14) ORR was 90% (14% CR). With a median follow up of 10 months, 35% (n=72) of venetoclax treated patients have discontinued therapy, and median PFS/OS have not been reached (Figure 1). The top three reasons for discontinuation were CLL progression (47%), Richter's transformation (21%), and venetoclax toxicity (11%), most commonly hematologic. Subset univariate analyses identified pre-venetoclax risk factors for inferior PFS (Table 2). Most common post-venetoclax therapies were KI (47.6%, n=19), rituximab monotherapy (12.5%, n=5), cellular therapy (12.5%, n=5). For the subset treated with KI therapy, ORR was 69% and PFS not reached (median follow up 7 months).
Conclusions: In the largest cohort of venetoclax-treated CLL patients, the majority successfully completed and maintained a maximum daily dose of 400 mg. ORRs and CRs appear comparable to clinical trial data. Venetoclax was active in patients with mutations known to confer ibrutinib resistance. Prior KI exposure and poor risk genetics were associated with shorter PFS. Hematologic toxicities were the most common AEs, and progression was the leading cause of venetoclax discontinuation. Although 76% were low-intermediate risk for TLS, hospitalization and rasburicase use to minimize perceived TLS risk were frequently employed. This report demonstrates responses to KIs following venetoclax discontinuation. Optimal sequencing of newer CLL therapies requires further study and additional analyses are planned.
Mato: Pharmacyclics: Research Funding; AbbVie: Consultancy, Research Funding; DTRM: Research Funding; AstraZeneca: Consultancy; Acerta: Research Funding; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Portola: Research Funding; Regeneron: Research Funding; Janssen: Consultancy; Kite: Consultancy; TG Therapeutics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Gilead Sciences, Inc.: Membership on an entity's Board of Directors or advisory committees. Tam: Janssen Cilag: Honoraria, Research Funding; Roche: Honoraria, Research Funding; Abbvie: Honoraria, Research Funding. Brander: Genentech: Consultancy; Teva Pharmaceuticals, Genentech, AbbVie, Pharmacyclics: Consultancy; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees; TG Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees. Pagel: Gilead: Consultancy; Pharmacyclics: Consultancy. Ujjani: Genentech: Consultancy; Abbvie: Research Funding, Speakers Bureau; Gilead: Consultancy; Pharmacyclics: Consultancy, Research Funding. Lamanna: Gilead: Membership on an entity's Board of Directors or advisory committees, Research Funding. Lansigan: Seattle Genetics: Consultancy; Spectrum Pharmaceuticals: Consultancy, Research Funding. Shadman: Acerta Pharma: Research Funding; Celgene: Research Funding; AbbVie: Other: advisory board; Genentech: Consultancy, Research Funding; TG Therapeutics: Research Funding; Merck: Research Funding; PLEXXIKON: Research Funding; Gilead: Research Funding; Emergent: Research Funding; Pharmacyclics: Other: advisory board, Research Funding. Skarbnik: Seattle Genetics: Speakers Bureau; Genentech: Speakers Bureau; Gilead: Speakers Bureau; Abbvie: Other: Ad board, Speakers Bureau; Novartis: Speakers Bureau. Barr: Seattle Genetics: Consultancy; Infinity: Consultancy; Gilead: Consultancy; Celgene: Consultancy; Novartis: Consultancy; Pharmacyclics LLC, an AbbVie Company: Consultancy, Research Funding; AbbVie: Consultancy, Research Funding. Cheson: Acerta, Pharmacyclics, Epizyme, Gilead, Roche, AbbVi: Other: Institution receives research support ; AbbVie, Roche-Genentech, Pharmacyclics, Acerta: Consultancy. Schuster: Celgene: Consultancy, Research Funding; Seattle Genetics: Consultancy; Gilead: Consultancy, Research Funding; Janssen: Consultancy, Honoraria; Bristol-Myers Squibb: Consultancy, Research Funding; Genentech: Consultancy, Research Funding; Merck: Research Funding; Nordic Nanovector: Consultancy; Novartis: Consultancy, Research Funding. Shah: Jazz Pharmaceuticals: Consultancy; Exelixis: Equity Ownership; Oncosec: Equity Ownership; Geron: Equity Ownership. Dorsey: TG Therapeutics, Inc.: Consultancy. Svoboda: BMS: Consultancy, Research Funding; Seattle Genetics: Consultancy, Research Funding; Pharmacyclics: Research Funding; Merck: Research Funding; Celgene: Research Funding; Kite: Consultancy. Furman: Sunesis: Consultancy; Gilead: Consultancy; Abbvie: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Genentech: Consultancy; Pharmacyclics: Consultancy, Honoraria; TG Therapeutics: Consultancy; Verastem: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal